查看完整版本请点击这里:
资料:Atomic absorption spectrometry
Atomic absorption spectrometry (AAS) is an analytical technique that measures the资料:Atomic absorption spectrometry
concentrations of elements. Atomic absorption is so sensitive that it can measure down to parts per billion of a gram (μg dm–3) in a sample. The technique makes use of the wavelengths of light specifically absorbed by an element. They correspond to the energies needed to promote electrons from one energy level to another, higher, energy level.
Atomic absorption spectrometry has many uses in different areas of chemistry.
Clinical analysis. Analysing metals in biological fluids such as blood and urine.
Environmental analysis. Monitoring our environment – eg finding out the levels of various
elements in rivers, seawater, drinking water, air, petrol and drinks such as wine, beer and fruit drinks.
Pharmaceuticals. In some pharmaceutical manufacturing processes, minute quantities of a catalyst used in the process (usually a metal) are sometimes present in the final product. By using AAS the amount of catalyst present can be determined. Industry. Many raw materials are examined and AAS is widely used to check that the major elements are present and that toxic impurities are lower than specified – eg in concrete, where calcium is a major constituent, the lead level should be low because it is toxic.
Mining. By using AAS the amount of metals such as gold in rocks can be determined to see whether it is worth mining the rocks to extract the gold.
How it works
Atoms of different elements absorb characteristic wavelengths of light. Analysing a sample to see if it contains a particular element means using light from that element. For example with lead, a lamp containing lead emits light from excited lead atoms that produce the right mix of wavelengths to be absorbed by any lead atoms from the sample. In AAS, the sample is atomised – ie converted into ground state free atoms in the vapour state – and a beam of electromagnetic radiation emitted from excited lead atoms is passed through the vaporized sample. Some of the radiation is absorbed by the lead
atoms in the sample. The greater the number ofatoms there is in the vapour, the more radiation is absorbed. The amount of light absorbed is proportional to the number of lead atoms. A calibration curve is constructed by running several samples of known lead concentration under the same conditions as the unknown. The amount the standard absorbs is compared with the calibration curve and this enables the calculation of the lead
concentration in the unknown sample.
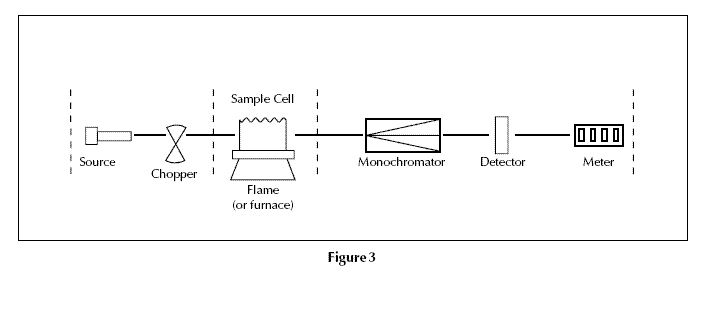
Consequently an atomic absorption spectrometer needs the following three components: a light source; a sample cell to produce gaseous atoms; and a means of measuring the specific light absorbed.
The light source
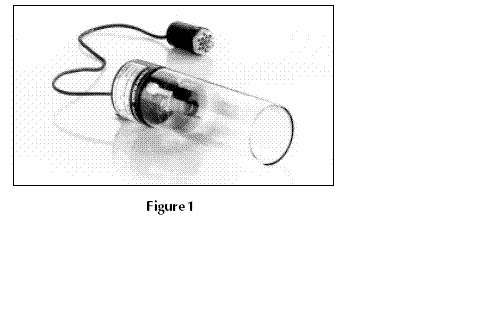
The common source of light is a ‘hollow cathode lamp’ (Fig. 1). This contains a tungsten anode and a cylindrical hollow cathode made of the element to be determined. These are sealed in a glass tube filled with an inert gas – eg neon or argon – at a pressure o
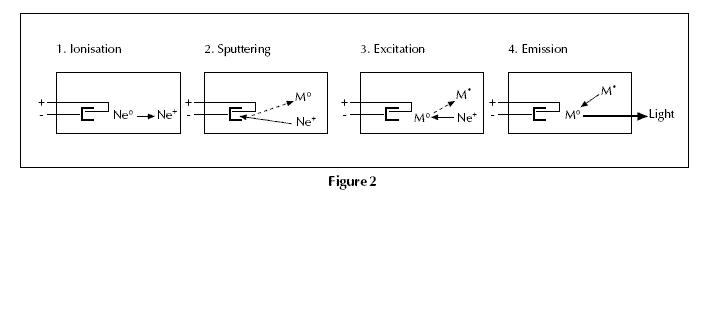
between 1 Nm–2 and 5 Nm–2. The ionisation of some gas atoms occurs by applying a potential difference of about 300–400 V between the anode and the cathode. These gaseous ions bombard the cathode and eject metal atoms from the cathode in a process
called sputtering. Some sputtered atoms are in excited states and emit radiation characteristic of the metal as they fall back to the ground state – eg Pb* → Pb + h (Fig. 2). The shape of the cathode concentrates the radiation into a beam which passes through a quartz window, and the shape of the lamp is such that most of the sputtered atoms
are redeposited on the cathode.
A typical atomic absorption instrument holds several lamps each for a different element. The lamps are housed in a rotating turret so that the correct lamp can be quickly selected.
The optical system and detector A monochromator is used to select the specific wavelength of light – ie spectral line – which is absorbed by the sample, and to exclude other wavelengths. The selection of the specific light allows the determination of the selected element in the presence of others. The light selected by the monochromator is directed onto a detector that is typically a photomultiplier tube. This produces an electrical signal proportional to the light intensity(Fig. 3).
Double beam spectrometers Modern spectrometers incorporate a beam splitter so that one part of the beam passes through the sample cell and the other is the reference (Fig. 4). The intensity of the light source may not stay constant during an analysis. If only a single beam is used to pass
through the atom cell, a blank reading containing no analyte (substance to be analysed) would have to be taken first, setting the absorbance at zero. If the intensity of the source changes by the time the sample is put in place, the measurement will be inaccurate. In the double beam instrument there is a constant monitoring between the reference beam and the light source. To ensure that the spectrum does not suffer from loss of sensitivity, the beam splitter is designed so that as high a proportion as possible of the energy of the lamp beam passes through the sample.
Atomisation of the sample Two systems are commonly used to produce atoms from the sample. Aspiration involves sucking a solution of the sample into a flame; and electrothermal atomisation is where a drop of sample is placed into a graphite tube that is then heated electrically.
Some instruments have both atomisation systems but share one set of lamps. Once the appropriate lamp has been selected, it is pointed towards one or other
atomisation system.
Flame aspiration
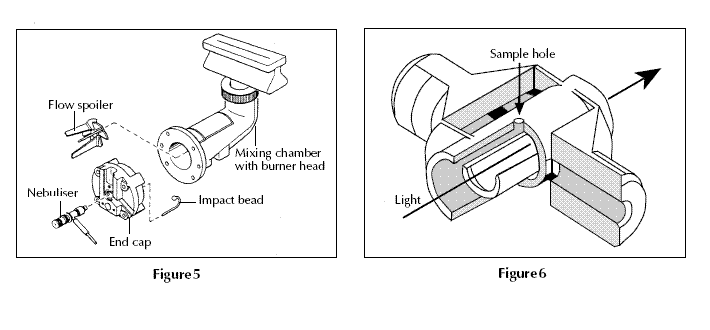
Figure 5 shows a typical burner and spray chamber.
Ethyne/air (giving a flame with a temperature of 2200–2400 °C) or ethyne/dinitrogen oxide (2600– 2800 °C) are often used. A flexible capillary tube connects the solution to the nebuliser. At the tip of the capillary, the solution is ‘nebulised’ – ie broken into small drops. The larger drops fall out and drain off while smaller ones vaporise in the flame. Only ca 1% of the sample is nebulised.
Electrothermal atomisation
Figure 6 shows a hollow graphite tube with a platform. 25 μl of sample (ca 1/100th of a raindrop) is placed through the sample hole and onto the platform from an automated micropipette and sample changer.
The tube is heated electrically by passing a current through it in a pre-programmed series of steps. The details will vary with the sample but typically they might be 30–40 seconds at 150 °C to evaporate the solvent, 30 seconds at 600 °C to drive off any volatile organic material and char the sample to ash, and with a very fast heating rate (ca 1500 °C s-1) to 2000–2500 °C for 5–10 seconds to vaporise and atomise elements (including the element being analysed).
Finally heating the tube to a still higher temperature– ca 2700 °C – cleans it ready for the next sample. During this heating cycle the graphite tube is flushed with argon gas to prevent the tube burning away. In electrothermal atomisation almost 100% of the sample is atomised. This makes the technique much more sensitive than flame AAS.
[ 本帖最后由 popshengu 于 2010-8-3 15:26 编辑 ]
查看完整版本请点击这里:
资料:Atomic absorption spectrometry
资料:Atomic absorption spectrometry



最新回复
popshengu (2010-8-03 15:27:24)
Sample preparation is often simple, and the chemical form of the element is usually unimportant. This is because atomisation converts the sample into free
atoms irrespective of its initial state. The sample is weighed and made into a solution by suitable dilution. Elements in biological fluids such as urine and blood are often measured simply after a dilution of the original sample. Figure 7 shows a flame atomic absorption spectrometer with an autosampler and flow injection accessory.
When making reference solutions of the element under analysis, for calibration, the chemical environment of the sample should be matched as closely as possible – ie the analyte should be in the same compound and the same solvent. Teflon
containers may be used when analysing very dilute solutions because elements such as lead are sometimes leached out of glass vessels and can affect the results.
Background absorption
It is possible that other atoms or molecules apart from those of the element being determined will absorb or scatter some radiation from the light source. These
species could include unvaporised solvent droplets, or compounds of the matrix (chemical species, such as anions, that tend to accompany the metals being
analysed) that are not removed completely. This means that there is a background absorption as well as that of the sample.
One way of measuring and correcting this background absorption is to use two light sources, one of which is the hollow cathode lamp appropriate to the element being measured. The second light source is a deuterium lamp.
The deuterium lamp produces broad band radiation, not specific spectral lines as with a hollow cathode lamp. By alternating the measurements of the two light sources – generally at 50 –100 Hz – the total absorption (absorption due to analyte atoms plus background) is measured with the specific light from the hollow cathode lamp and the background absorption is measured with the light from the
deuterium lamp. Subtracting the background from the total absorption gives the absorption arising from only analyte atoms.
Calibration
A calibration curve is used to determine the unknown concentration of an element – eg lead – in a solution.
The instrument is calibrated using several solutions of known concentrations. A calibration
curve is produced which is continually rescaled as more concentrated solutions are used – the more concentrated solutions absorb more radiation up to a certain absorbance. The calibration curve shows the concentration against the amount of radiation absorbed (Fig. 8(a)).
The sample solution is fed into the instrument and the unknown concentration of the element – eg lead – is then displayed on the calibration curve (Fig. 8(b)).
5.gif
A bad paint job
Atomic absorption spectrometry is sometimes used for investigating unusual problems. One such case was that of a seriously ill baby whose symptoms could not be explained.
Lead is a toxic element that can cause poisoning in children. A baby was brought to a hospital suffering from vomiting and stomach pains, and was very drowsy. There were no obvious reasons or signs why the child should be ill.
As part of the routine tests performed, the lead level in a blood sample from the child was measured using electrothermal atomisation AAS. The lead level was higher than normal and there was no known source for the lead. However, the parents explained that the child had been chewing the painted wood on its cot. The paint was also examined by dissolving it in nitric acid and then using flame AAS to find out the lead content. A very high level was found.
Other paints in the baby’s bedroom were found to have low lead levels. This identified the cot paint as the source of lead in the baby. The baby’s cot was old and had been painted when leaded paint was very common. This type of paint is now banned from household use and by law all painted toys must be examined for lead and other toxic metals to make sure that they are safe for small children.
资料:Atomic absorption spectrometry